MASS DRUG ADMINISTRATION
BackLymphatic Filariasis Elimination Goal
The Government of India is signatory to the World Health Assembly Resolution in 1997 for Global Elimination of Lymphatic Filariasis. The National Health Policy (2002) envisages elimination of lymphatic filariasis in India by 2015.
Strategy for Elimination of Lymphatic Filariasis
- Annual Mass Drug Administration (MDA) of single dose of DEC (Diethylcarbamazine citrate) and Albendazole for 5 years or more to the eligible population (except pregnant women, children below 2 years of age and seriously ill persons) to interrupt transmission of the disease.
- Home based management of lymphoedema cases and up-scaling of hydrocele operations in identified CHCs/ District hospitals /medical colleges.
Progress and Achievement
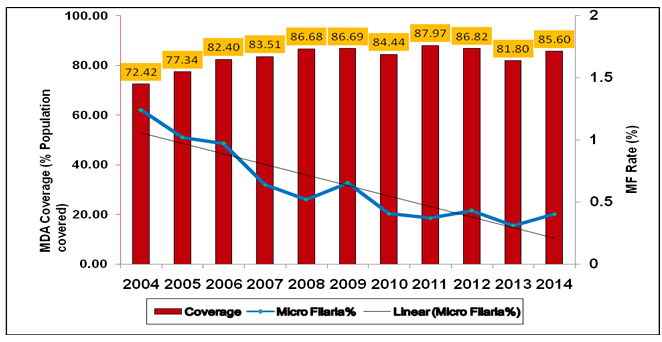
In pursuit of the goals, the Government of India launched nationwide MDA in 2004 in endemic areas as well as home based morbidity management, scaling up hydrocelectomies in hospitals and CHCs. During the year 2004, only 202 districts could be covered with coverage rate of 72.6%. The number of districts was upscaled and in 2007 all the 250 known LF endemic districts were brought under MDA. The policy decision to implement global strategy of co-administration of DEC with Albendazole during MDA was approved by National Task Force on Elimination of Lymphatic Filariasis under the Chairmanship of DGHS. The population coverage during MDA has improved from 73% in 2004 to 83% in 2013 (Prov.) which has resulted in the overall reduction of microfilaria rate from 1.24% in 2004 to 0.29% in 2013 (Prov.)
Capacity building has improved the performance of various functionaries. The initiative was taken to involve senior faculties from various medical colleges during 2005-2007. A total of 544 faculty members belonging to medicine, community medicine, pharmacology, microbiology and paediatrics were trained from 79 medical colleges. Subsequently trainings were imparted in state and approximately about 1 million health personnels including Medical Officers, Paramedics, Drug Distributors, Lab. Technicians, etc are trained annually on MDA and Morbidity management.
Intensive social mobilization during MDA, have been carried out by various states/ UTs involving political/ opinion leaders, decision makers, local leaders and community.
Validation through Transmission Assessment Survey (TAS)
All the districts have completed more than 5 rounds of MDA by the end of 2014, and are required to be evaluated to decide whether to stop or continue MDA. As per WHO guidelines-2011, the districts having observed minimum five rounds of MDA with more than 65% coverage against total population at risk in implementation unit (population of district covered under MDA) are to be subjected for Transmission Assessment Survey (TAS) using Immuno-chromatographic test (ICT) for presence of circulating antigenaemia in children born after initiation of MDA to know the current infection. During July 2012, WHO conducted a Regional Workshop on Capacity Building on TAS at Puducherry (India) for all Member countries of SEAR.
- Afterwards four National level Trainer’s Training Workshops have been organized for which financial support was provided by WHO. Workshops were conducted at Pune, Bhubaneswar, Chennai and Bangalore. ICMR, NCDC and WHO Officers were also involved during the training. In these workshops, a total of 139 state and district level officials as a result of which the capacity of district level officials were improved and till May, 2015, 49 districts with 66 evaluation units (approv. 2 million population each) have been successfully cleared through TAS. The details of the 49 districts are (5 of Assam, 2 of Goa, 5 of Gujarat, 3 of Karnataka, 4 of Kerala, 4 of Maharashtra, 16 of Tamilnadu, 4 of Odisha, 4 of West Bengal and 1 each of Daman & Diu and Puducherry) have successfully completed TAS exercise and qualified for MDA stoppage.
- During 2015-16, TAS is expected to be carried out in 68 districts (6 of Andhra Pradesh, 2 of Telangana, 2 of Assam, 10 of Bihar, 3 of Chattisgarh, 1 of Gujarat, 3 of Jharkhand, 1 of Karnataka, 4 of Kerala, 2 of Madhya Pradesh, 2 of Maharashtra, 5 of Odisha, 4 of Tamil Nadu, 18 of Uttar Pradesh, 2 of West Bengal, 1 each of A&N Island, Dadra & Nagar Haveli and Lakshadweep). Majority districts are waiting for procurement and supply of ICT cards which is manufactured by only one company i.e. Allere (Binax), USA. In July, 2015, a National level trainer’s training workshop on TAS with demonstration of ICT and FST (Filaria Strip Test) was organized at VCRC, ICMR, Puducherry with the support of WHO for 30 officials from different states, NVBDCP headquarter, Regional offices for health & F.W. and medical college. Five more such workshops at state level for district level officers are proposed. The list of participants trained in 2013 and 2015 on TAS may be seen.
Morbidity Management and Disability Alleviation
- Morbidity Management is another pillar of strategy for ELF and states/UTs were advised on up-scaling home based morbidity management of Lymphoedema cases and Hydrocele operations. The process involved updating the line-listing of Lymphoedema & Hydrocele cases in the districts. Demonstration and training on simple foot hygiene to affected persons and motivate them for self practice. Motivate for surgical intervention to hydrocele cases. The updated report from LF endemic states/UTs indicated 8.27 lakh Lymphoedema and 3.76 lakh hydrocele cases
- Since 2004, the states/UTs have reported 129572 hydrocele operations. Different states have initiated management of Lymphodema cases through demonstrating home based foot hygiene method to patients at local levels.